
在DMAP催化下,以DCC为偶联试剂的酯化方法。1978年Steglich首先提出【Angew. Chem. Int. Ed.1978,17,522】,该方法条件温和,可用于位阻大的或对酸敏感底物的酯化,适用于从叔丁醇制备叔丁酯。而传统的Fischer酯化法(酸催化酯化)会导致叔丁醇消除。该法也可用于硫代酸酯的合成。
Keck在研究用此方法合成大环内酯时,发现加入DMAP.HCl可以提高质子转移效率,提高酯化收率。【J. Org. Chem. 1985, 50, 2394】
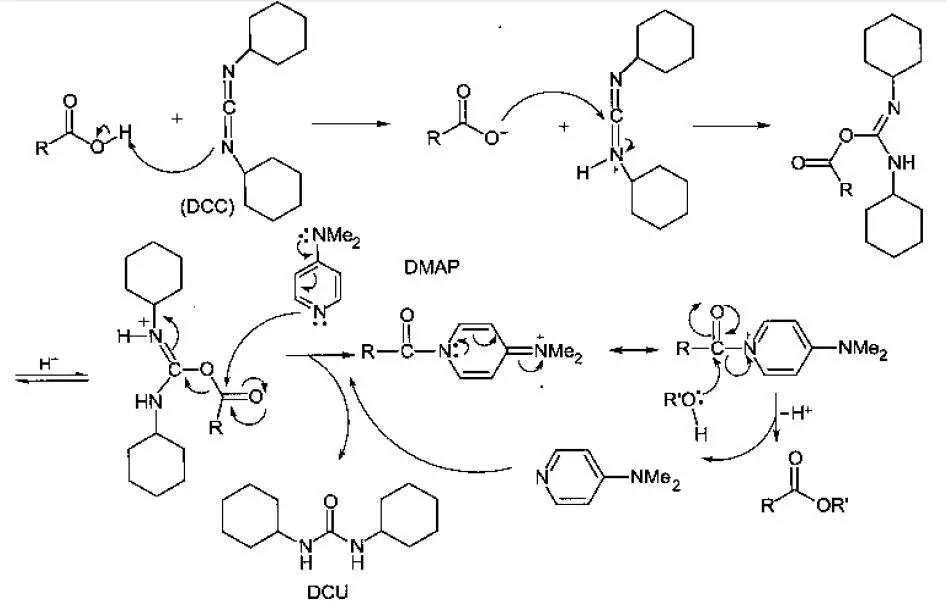
反应机理
首先羧酸先和DCC反应生成活性酯,接着和DMAP交换生成活性酰胺,醇进攻活性酰胺,生成酯。

此方法合成天然产物hapalosin,产率比Yamguchi法(Yamaguchi酯化反应)产率高。【J. Comb. Chem. 2007, 9, 386】

A 500-mL, one-neckedflask equipped with a calcium chloride drying tube was charged with 28.83 g(0.20 mol) of monoethyl fumarate, 200 mL of dry dichloromethane, 44.47 g (0.60mol) of tert-butyl alcohol, and 2.00 g (0.16 mol) of 4-dimethylaminopyridine.The solution was stirred and cooled in an ice bath to 0°C while 45.59 g (0.22mol) of dicyclohexylcarbodiimide was added over a 5-min period. After a further5 min at 0°C the ice bath was removed and the dark-brown reaction mixture wasstirred for 3 h at room temperature. The dicyclohexylureathat has precipitated was removed by filtration through a fritted Büchnerfunnel (G3), and the filtrate was washed with two 50-mL portions of 0.5 Nhydrochloric acid and two 50 mL portions of saturated sodium bicarbonatesolution. During this procedure some additional dicyclohexylurea wasprecipitated, which was removed by filtration of both layers to facilitatetheir separation. The organic solution was dried over anhydrous sodium sulfateand concentrated with a rotary evaporator. The concentrate was distilled underreduced pressure, affording after a small forerun, 30.5–32.5 (76–81%) oftert-butyl ethyl fumarate, bp 105–107°C (12 mm)。
【Organic Syntheses, Coll. Vol. 7, p.93 (1990); Vol. 63, p.183 (1985)】
编辑自:《有机人名反应、试剂与规则》,黄培强等。
 )
)






























我来说两句排行榜